The DECISION project – European researchers seek to reduce the number of patients dying from cirrhosis
21 European institutions join forces to tackle end-stage liver disease and liver failure with a systems medicine approach
21 European institutions join forces to tackle end-stage liver disease and liver failure with a systems medicine approach
- Despite a vast array of available interventions and medications, more than 1 million people die of chronic liver disease (cirrhosis) per year worldwide, when the disease progresses to decompensated cirrhosis and acute-on-chronic liver failure (ACLF), a state in which the dysfunctional liver induces failure of other organs.
- Following an acute decompensation of cirrhosis, 14% of the patients die of ACLF within 3 months. The reason why certain patients die and others survive is unknown, but huge differences between patients with regard to their individual genetics, medical history, precipitating events, clinical presentation and treatment response are suspected.
- These individual differences call for personalised treatments based on a precise understanding of underlying mechanisms. Systems medicine and high-throughput technology nowadays allow for highly efficient analysis, integration, and predictive modelling of clinical data to develop the best fitted, most personalised treatment for each patient.
- Over the next 5.5 years, the DECISION research consortium will analyse and integrate data from already existing clinical data and biological samples from 2,200 patients with cirrhosis at more than 8,600 time points to identify novel combinatorial therapies, validate them in animal models, and then test the most promising combinatorial therapy in a clinical trial.
- The overall aim of the DECISION project is to prevent ACLF and to significantly reduce the mortality rate amongst patients with decompensated cirrhosis. The project receives 6 million € funding from the European Commission.
Why is decompensated cirrhosis fatal?
End-stage chronic liver disease (cirrhosis) is a major cause of morbidity and mortality, and has a large socioeconomic impact because of high health care costs and the patients’ inability to work or seek employment. Patients show symptoms, start suffering, and eventually die of chronic liver cirrhosis when the body essentially can’t compensate the dysfunctional liver condition any longer. That’s why it’s called decompensated (as opposed to compensated) cirrhosis. Decompensated cirrhosis is defined by accumulation of fluid in the abdomen (ascites), impaired brain function (hepatic encephalopathy), and often also bleeding in the digestive tract (gastrointestinal haemorrhage). Eventually, it progresses to acute-on-chronic liver failure (ACLF) and death.
Despite a vast array of available treatments for decompensated cirrhosis, such as albumin, antibiotics and antiviral agents, anticoagulants, beta-blockers, diuretics, laxatives, proton-pump inhibitors, statins, steroids, and vasoconstrictors, 5% of the patients die by day 28, 14% within 90 days. Research suggests individual differences between patients with regard to genetics, precipitating events and treatment response as the main culprit for the high mortality rate. This heterogeneity calls for better patient stratification and treatment personalization based on underlying biological mechanisms.
Despite a vast array of available treatments for decompensated cirrhosis, such as albumin, antibiotics and antiviral agents, anticoagulants, beta-blockers, diuretics, laxatives, proton-pump inhibitors, statins, steroids, and vasoconstrictors, 5% of the patients die by day 28, 14% within 90 days. Research suggests individual differences between patients with regard to genetics, precipitating events and treatment response as the main culprit for the high mortality rate. This heterogeneity calls for better patient stratification and treatment personalization based on underlying biological mechanisms.
How will patients with cirrhosis benefit from DECISION?
After identifying the most promising novel combinatorial treatment approaches via data analysis and predictive mathematical modelling in silico, DECISION researchers will first refine and optimise these therapies in animal models. Subsequently, the best combinatorial therapy will be tested in patients with cirrhosis in a phase II clinical trial. In addition, the DECISION consortium will develop two novel tests aiding hepatologists in everyday clinical decision making: one to reliably predict the therapeutic outcome in patients with decompensated cirrhosis when treated with standard treatment (prognostic test), the other one to successfully identify those patients who will respond best to the novel combinatorial therapy (test for predicted response). Thus, the patients participating in these clinical studies may directly benefit from DECISION research, while future patients with cirrhosis will hopefully benefit from novel combinatorial therapies and improved clinical guidelines derived from the project’s findings and results.
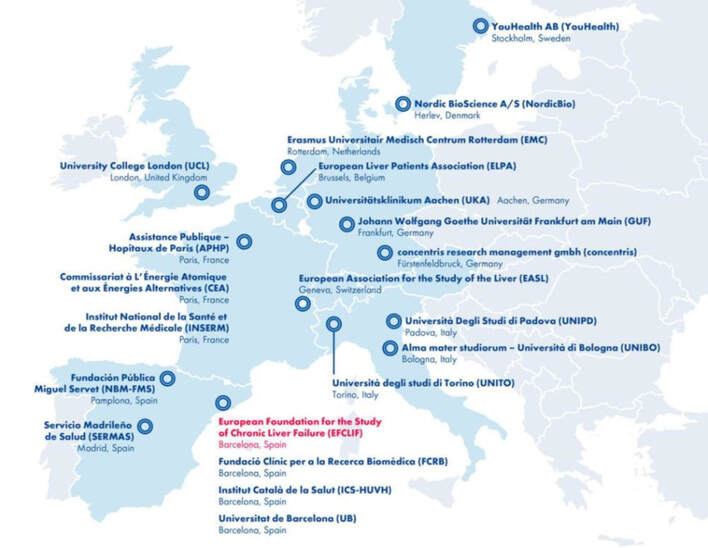
Who are the partners of the DECISION research consortium?
Professor Pierre-Emmanuel Rautou (MD, PhD), member of the European Foundation for The Study of Chronic Liver Failure (EFCLIF), Barcelona, and professor for hepatology at the French Université de Paris (APHP) and the French Institut national de la santé et de la recherche médicale (INSERM), spearheads and coordinates DECISION. The 21 institutions that collaborate in this multi-centre project are spread throughout Europe and include hepatologists, molecular biologists, systems medicine specialists and patients’ association. The multidisciplinary team strives for high-impact dissemination of scientific results and improved clinical guidelines:
1. Alma Mater Studiorum – Universita di Bologna (UNIBO), Italy
2. Assistance Publique Hôpitaux de Paris (APHP) and University of Paris, France
3. Commissariat à l’Energie Atomique et aux Energies Alternatives (CEA), France
4. concentris research management GmbH (concentris), Germany
5. Erasmus Universitair Medisch Centrum Rotterdam (EMC), Netherlands
6. European Association for the Study of the Liver (EASL), Switzerland
7. European Foundation for the Study of Chronic Liver Failure (EFCLIF), Spain
8. European Liver Patients Association (ELPA), Belgium
9. Fundació Clínic per a la Recerca Biomèdica (FCRB), Spain
10. Fundacion Publica Miguel Servet (NBM-FSM), Spain
11. Institut Catala de la Salut (ICS-HUVH), Spain
12. Institut national de la santé et de la recherche médicale (INSERM), France
13. Johann Wolfgang Goethe-Universität Frankfurt Am Main (GUF), Germany
14. Nordic Bioscience A/S (NordicBio), Denmark
15. Servicio Madrileno de Salud (SERMAS), Spain
16. Universita degli Studi di Padova (UNIPD), Italy
17. Universita degli Studi di Torino (UNITO), Italy
18. Universitätsklinikum Aaachen (UKA), Germany
19. Universitat de Barcelona (UB), Spain
20. University College London (UCL), United Kingdom
21. YH YouHealth AB (YouHealth), Sweden
1. Alma Mater Studiorum – Universita di Bologna (UNIBO), Italy
2. Assistance Publique Hôpitaux de Paris (APHP) and University of Paris, France
3. Commissariat à l’Energie Atomique et aux Energies Alternatives (CEA), France
4. concentris research management GmbH (concentris), Germany
5. Erasmus Universitair Medisch Centrum Rotterdam (EMC), Netherlands
6. European Association for the Study of the Liver (EASL), Switzerland
7. European Foundation for the Study of Chronic Liver Failure (EFCLIF), Spain
8. European Liver Patients Association (ELPA), Belgium
9. Fundació Clínic per a la Recerca Biomèdica (FCRB), Spain
10. Fundacion Publica Miguel Servet (NBM-FSM), Spain
11. Institut Catala de la Salut (ICS-HUVH), Spain
12. Institut national de la santé et de la recherche médicale (INSERM), France
13. Johann Wolfgang Goethe-Universität Frankfurt Am Main (GUF), Germany
14. Nordic Bioscience A/S (NordicBio), Denmark
15. Servicio Madrileno de Salud (SERMAS), Spain
16. Universita degli Studi di Padova (UNIPD), Italy
17. Universita degli Studi di Torino (UNITO), Italy
18. Universitätsklinikum Aaachen (UKA), Germany
19. Universitat de Barcelona (UB), Spain
20. University College London (UCL), United Kingdom
21. YH YouHealth AB (YouHealth), Sweden
Caption: The European Foundation for the Study of Chronic Liver Failure (EFCLIF, in red) coordinates the DECISION project, a 5.5 year-long Horizon 2020-funded research project with 21 partner sites across Europe.
VISIT THE WEBSITE CLICK HERE
VISIT THE WEBSITE CLICK HERE
Paragraph. Cliquer ici pour modifier.